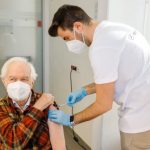
Researchers at the University of Washington are looking for volunteers to participate in a COVID booster vaccine trial.
Researchers from the University of Washington need volunteers for a COVID booster vaccine trial.
University of Washington School of Medicine
The trial, led by the UW’s School of Medicine in Seattle, will test a participant’s immune response to the Booster dose shot
specifically to test its “safety and tolerability,” according to a news release from the medical school.
The goal of the trial is to “elicit an immune response to multiple SARS-CoV-2 proteins in addition to the spike protein that is targeted by currently available vaccines made by Moderna, Pfizer and Johnson & Johnson.”
In targeting multiple proteins, researchers said they hope the Booster dose will protect people from a variety of COVID strains and variants.
“With the emergence of the Delta and other COVID-19 variants, we need to stay ahead of the virus by developing effective vaccines that will aid in the prevention of all strains of COVID,” Director of the UW Medicine Virology Research Clinic and head the UW School of Medicine’s allergies and infectious diseases division Dr. Anna Wald said.
Who can enroll?
- Anyone 18 years and older
- Fully vaccinated four months before the enrollment
- Healthy
- Without significant allergies
- Hasn’t had COVID-19 before
- If someone meets all of the qualifications, they are eligible for the vaccine trial.
What will be asked of participants?
- Attend nine to 14 in-person clinic visits and one to two phone check-ins over the course of 12 to 14 months
- Receive one or two shots of the vaccine
- Blood will be drawn “several times” to monitor immune response and safety of the vaccine
- Record how one is feeling after the shot
“We hope that these investigational vaccines enhance and broaden the immune response elicited by vaccines currently available in the U.S.,” said Dr. Tia Babu, acting assistant professor at the University of Washington School of Medicine.
Schools in Texas, Missouri and Georgia are also conducting Booster dose vaccine trials.
- Decatur, Georgia: Emory Vaccine Center, The Hope Clinic
- St. Louis: Saint Louis University, Center for Vaccine Development
- Houston: Baylor College of Medicine, Molecular Virology and Microbiology
also read :
- Moderna, Pfizer COVID-19 vaccine hesitancy: Does the data support concerns about side effects?
- Pfizer’s COVID Vaccine Gets Full Approval From The FDA
- Schools Reopen to Mask Confusion ? CDC mask mandate | news | COVID-19
Volunteers sought for vaccine trial of Covid Booster dose
The trial seeks to test the safety and tolerability of an experimental vaccine.
Researchers at the University of Washington School of Medicine are enrolling volunteers for a COVID-19 booster vaccine trial.
Volunteers will participate in the second stage of a phase 1 vaccine trial.
Phase 1 vaccine trials are designed to test the safety and tolerability of and immune response to a new vaccine.
In the first stage of the trial, the experimental vaccines were given to unvaccinated volunteers. In this second stage, the vaccines will be given as a booster shot to volunteers who have already been vaccinated against SARS-CoV-2.
Unlike current vaccines, the trial vaccines seek to elicit an immune response to multiple SARS-CoV-2 proteins in addition to the spike protein that is targeted by currently available vaccines made by Moderna, Pfizer and Johnson & Johnson.
The hope is that by targeting a number of coronavirus proteins, the vaccine will provide protection against a wide variety of SARS-CoV-2 strains and variants. The vaccine candidates were developed by Gritstone bio, headquartered in Emeryville, CA.
“With the emergence of the Delta and other COVID-19 variants, we need to stay ahead of the virus by developing effective vaccines that will aid in the prevention of all strains of COVID,” said Dr. Anna Wald, director of the UW Medicine Virology Research Clinic and head the UW School of Medicine’s allergies and infectious diseases division.
She is the trial site principal investigator.
“We hope that these investigational vaccines enhance and broaden the immune response elicited by vaccines currently available in the U.S.,” said Dr. Tia Babu, acting assistant professor at the University of Washington School of Medicine and a trial investigator.
To enroll, participants must be age 18 or older, healthy, without significant allergies, without a history of prior SARS-CoV-2 infection and have been vaccinated against COVID-19 at least four months prior to enrollment. Persons over age 60 are encouraged to participate.
Participants will be asked to:
- Make nine to 14 or more in-person clinic visits and also will receive one to two telephone check-ins with study staff over 12 to 14 months.
- Receive one or two injections of investigational vaccine.
- Have blood drawn several times to monitor safety and to see whether the vaccine results in an immune response.
- Keep track of how they’re feeling after the injection.
Booster dose of COVID-19 vaccines not needed yet
Volunteers needed for UW Medicine COVID-19 booster trial
Researchers at the University of Washington School of Medicine need volunteers for a COVID-19 booster trial.
Those who volunteer will take part in the second stage of a Phase 1 trial. It’s meant to test the safety, tolerability, and immune response to a new vaccine.
In the first stage of the trial, the experimental vaccines were given to unvaccinated volunteers. In this second stage, the vaccines will be given as a booster shot to volunteers who have already been vaccinated against the coronavirus.
The hope is that by targeting a number of coronavirus proteins, the vaccine will give protection against a wide variety of SARS-CoV-2 strains and variants.
The vaccine candidates were developed by Gritstone bio.
“With the emergence of the Delta and other COVID-19 variants, we need to stay ahead of the virus by developing effective vaccines that will aid in the prevention of all strains of COVID,” said Dr. Anna Wald, director of the UW Medicine Virology Research Clinic and head the UW School of Medicine’s allergies and infectious diseases division. She is the trial site’s principal investigator.
“We hope that these investigational vaccines enhance and broaden the immune response elicited by vaccines currently available in the U.S.,” said Dr. Tia Babu, acting assistant professor at the University of Washington School of Medicine and a trial investigator.
5 reasons why FDA advisers did not recommend Covid-19 booster shots for everyone
(CNN) Vaccine advisers to the US Food and Drug Administration declined Friday to recommend the agency approve Covid-19 booster doses for everyone who got vaccinated six months ago or longer.
They did recommend a more limited step: emergency use authorization for people 65 and older, and for people at high risk of severe infection. Then they went back and added in health care workers and other people at high risk of getting infected at work — even if they are not at especially high risk of severe disease.
But why not just go ahead and say everyone who wants a booster can get one? Members of the Vaccines and Related Biological Products Advisory Committee were effusively vocal about why not.
They think it’s too soon
“The stated goal of this vaccine has been to protect against serious illness,” Dr. Paul Offit, a professor of pediatrics at the Children’s Hospital of Philadelphia, told the meeting. “Data shows that these vaccines do exactly that,” he added. “It’s exactly what you’d expect.”
Even Pfizer said its vaccine was still very much preventing severe disease, hospitalizations and deaths in the US, with an effectiveness of more than 90%. The company argued that this might not last much longer, but many members of VRBPAC didn’t buy it.
They don’t see enough evidence to justify booster shots for everyone
“It is my opinion that we need this in our armamentarium — a booster dose now, particularly for the elderly and (those with) other high-risk conditions — but I share my colleagues’ angst about the sparsity of safety data,” said Dr. Mark Sawyer, a professor of clinical pediatrics at the University of California San Diego.
“I am hopeful that CDC rolls this out in a gradual fashion.”
Dr. Michael Kurilla, an infectious disease specialist at the National Center for Advancing Translational Sciences, said he suspected Pfizer went too far in extrapolating data on older people to a younger population.
“So it’s unclear that everyone needs to be boosted, other than a subset of the population that clearly would be at high risk for serious disease,” Kurilla said. “It is not clear to me that the data we are seeing now is applicable to the general population.”